Taking a skin scrape
Taking and preparing a skin scrape involves using a blunt scraper, such as a wooden spatula, to gently take a sample of mucus from either immediately behind the gill cover, alongside the dorsal fin or the base of the tail.
The scraper is held at approximately 45 degrees to the body and drawn backwards towards the tail in a smooth movement, lifting off a small amount of mucus from the sample site. The mucus sample is then smeared onto a clean microscope slide along with a drop of pond water. Never use tap water as any residual chlorine could kill any parasites that are present!
The sample is then covered with a cover-slip and examined under the microscope, usually low-power, for the presence and number of parasites. I would normally take at least two samples, from different sites, from each fish being examined.
Taking a skin scrape and gill biopsy |
|
 Taking a skin scrape using a wooden spatula to gently lift off a mucus sample from just behind the operculum Taking a skin scrape using a wooden spatula to gently lift off a mucus sample from just behind the operculum |
|
 The mucus sample is put onto a glass microscope slide. A drop of pond water is added and a cover slip put on top The mucus sample is put onto a glass microscope slide. A drop of pond water is added and a cover slip put on top |
|
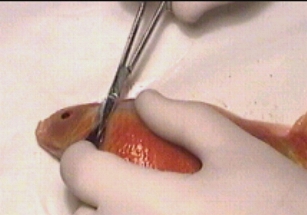 Taking a small gill biopsy using a fine pair of scissors Taking a small gill biopsy using a fine pair of scissors |
|
|
photos: Frank Prince-Iles |
|
Be careful!
There are certain considerations if the examination is to yield useful results and avoid causing damage to the fish being examined. The most important concern is to avoid injury to the fish during what should be a simple, safe procedure.
If the fish does have parasites they will be found in the mucous layer (though some will also penetrate the epidermis, e.g. ‘Ich’), so it is the mucous layer we need to sample for the wet-slide preparation. It is important to realise that damage caused to the epidermis while taking a mucus sample will be detrimental to the fish, so only gentle pressure with a blunt scraper should be applied – never use anything sharp that might damage the epidermis.
To sedate or not sedate – that is the question
Another consideration is whether the fish should be sedated while a scrape is taken. This is a subject that most books and magazines seem to avoid so I will put my neck on the block and give my thoughts on the subject.
In the first instance we want the fish to be still enough to do the scrape properly, while at the same time avoiding damage to the epidermis. With two people, one holding the fish and the other taking the scrape, it is possible to sample smaller fish and docile larger ones without the need for sedation. However, if there is likely to be a lot of flapping around, there is a real chance that mucus may be stripped off by the constant handling, thus giving an inaccurate result or, alternatively, a danger that the fish may be damaged.
On the other hand, if fish are routinely sedated prior to taking a scrape, there is the slight added risk that the fish could die from an overdose of anaesthetic. There is also the consideration as to whether the anaesthetic will affect the parasites and give a false result- although this has not been my experience if the scrape is taken and prepared quickly. In short, it seems to be a question of experience in deciding whether the procedure can be carried out effectively and safely without anaesthetic or whether the additional risk involved in sedating the fish is the lesser of two evils.
I must say that I have come across some rather inaccurate conclusions when people have tried to take scrapes from large, unsedated, lively fish! When dealing with larger koi on my own I invariably find MS222 anaesthetic a help. Your thoughts on this subject would be welcome!
Gill sample
If a parasitic infestation of the gill is suspected it is possible to sample mucus from the gill. It goes without saying that this procedure is potentially dangerous and must be carried out with extreme care and only on sedated fish.
A mucus sample can be taken from the gills by gently inserting a cotton-bud under the operculum and rolling it over the gill filaments. Under no circumstances should any pressure be exerted that may damage this vital and delicate structure. The mucus is then spread onto the microscope slide. For accurate results it is important that the sample is prepared and examined as quickly as possible.
With care it is also possible to take a small sample of gill using fine scissors to take a small biopsy from the lamellae tips. I urge that this procedure is not carried out unless one is totally confident about the procedure as the tiniest slip could cause considerable damage to the fish.
How many is too many?
Generally one or two observed parasites per slide should not be a reason for concern, whereas ten or more per field of view would be. Usually, when there is a serious infestation it is quite obvious as the slide often seems alive with parasites!
There are two potential problems that may be encountered when examining a skin or gill scrape. All of the smaller parasites are virtually transparent and unless the slide is scanned slowly and methodically there is always the possibility that some parasites may be missed, especially if they are not moving. If there seems to be a problem it is worth lowering the microscope condenser to improve contrast or, better still, if the option exists, try viewing the slide under dark-field.
If the mucus scrape is particularly thick it may be necessary to view at different levels, starting from the bottom and working progressively to the top. Otherwise, there is the chance that smaller parasites may be missed because they are hidden in the mucus. This becomes more likely at higher magnifications.
Don’t jump to conclusions
The second, more common, potential problem is simply jumping to conclusions. It is just too easy to spot the most obvious parasites, for example skin and gill flukes, and conclude that these are the problem. Even when you have spotted parasites it is vital that the whole slide is still methodically examined to make sure that nothing important is missed and so you build up to the correct conclusion. My own record is: five different species of parasite on one slide – but it took a full 15 minutes to find them all!
If a fish is found to be heavily infested it is worth taking a scrape from one or two others – to determine whether there is a general problem in the pond or if this is an isolated instance. It is often the case that rather than the whole pond requiring treatment, just the one fish is ill and the parasite explosion has resulted as a secondary infection for that fish, not the whole collection.
