A natural process that aids fish keepers
Nitrification is a biological process during which nitrifying bacteria convert toxic ammonia to less harmful nitrate. All we need to do is provide the right conditions for the nitrifying bacteria to thrive. It is important to bear in mind the potential threat to fish health if nitrification is affected.
Biological filtration and fish health
Nitrification is the natural biological process that lies at the heart of all fish filtration systems, with beneficial nitrifying bacteria converting toxic ammonia stepwise to less harmful nitrate. Without this constant water purifying activity most forms of fish keeping would be impossible, as fish would rapidly poison themselves on their own wastes. A salient point to bear in mind should anything interfere with or inhibit this process!
The main source of ammonia comes from metabolic ammonia excreted as a waste product from the gills. In summer temperatures koi will excrete between 50 – 100 mg ammonia per kilogram bodyweight daily – which is a lot of ammonia in a heavily stocked pond! The other ammonia sources come from fish waste, decomposing fish food and other organic matter such as algae etc.
From ammonia to nitrate
There two bacterial species involved. Nitrosomonas sp. bacteria which oxidize ammonia to nitrite, while Nitrobacter bacteria convert nitrite to nitrate, with both species utilising the energy released by the reactions. This seemingly simple process involves a complex series of reactions that can be summarised in chemist shorthand as:
-
For Nitrosomonas: 55NH4++ 76 O2 + 109HCO3–
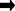 C5H7O2N + 54NO2–+ 57H2O + 104H2CO3
C5H7O2N + 54NO2–+ 57H2O + 104H2CO3 -
For Nitrobacter:400NO2– + NH4+ + 4H2CO3 + HCO3– + 195 O2
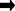 C5H7O2N + 3H2O + 400 NO3–
C5H7O2N + 3H2O + 400 NO3–
What these reactions tell us in plain language is that;
- in equation (1), ammonia (NH4+) is combined with oxygen and hydrogen carbonate to produce bacterial cell mass, nitrite (NO2–) , water and carbonic acid,
- in equation (2), nitrite is combined with ammonia, carbonic acid, hydrogen carbonate and oxygen to produce bacterial cell mass, water and lots of nitrate (NO3–).
While this all seems a bit grand and a bit unnecessary there are two important points that come out of these equations.
- Approximately 4.3 mg O2 are consumed for every mg of ammonia-nitrogen oxidised to nitrate-nitrogen
- 8.64 mg of alkalinity in the form ofHCO3– are consumed per mg of ammonia-nitrogen oxidised. This is quite a substantial amount of alkalinity and will over a period of time dramatically change the character of the pond water, affecting (decreasing) both hardness and pH stability. It is also an acidifying process, producing a gradual build up of nitric acid. It should also be noted that the process does not remove any nitrogen from the system; merely changing it from one form to another.
 |
nitrification uses substantial amounts of oxygen and carbonate, reducing water hardness and buffering capacity and has a mild acidifying affect. |
Nitrifiers:
The health and well being of these micro-friends should be almost as important to us as the health of our fish. For a vigorous growth of nitrifying bacteria we should endeavor to provide nature’s little helpers with the best conditions possible – after all without them we are likely to end up with lots of dead or sick fish. We need to be aware that nitrifiers are delicate organisms and extremely susceptible to a variety of inhibitors. They are extremely slow growing, unlike many bacteria that can double their numbers every hour or so. They are affected by a variety of organic and inorganic substances and have a very narrow pH tolerance, preferring a range between 7.5 – 8.6. Their growth rate is affected by temperature and they need a minimum of 1 mg/ litre of dissolved oxygen. Many chemical agents commonly used to treat fish diseases also affect them.
Where there’s muck
It is commonly believed that a filter has to be mucky to encourage the growth of nitrifying bacteria – wrong. If anything, this can have the opposite effect with faster-growing heterotrophic bacteria competing for resources such as oxygen. There is also the possibility that many of the decomposition products from the breakdown of rotting organic matter may inhibit nitrifying bacteria, either by direct toxicity or creating a hostile microenvironment.
In order to thrive, nitrifying bacteria only need a relatively clean environment with a steady supply of ammonia and oxygen. Indeed, it is possible to set up a fully functioning filtration system without any fish, just by putting a few drops of household ammonia in the water every day! I have kept filters running for several months this way.
So what about the nitrate?
Although nitrate does not represent a direct health threat to most fish, high levels are still undesirable. Apart from encouraging unsightly algal growth, it is now believed that high nitrate levels are implicated in some fish diseases. In nature the fate of most nitrate would be assimilation by plants to produce organic nitrogen compounds as part of the nitrogen cycle. Dare I say it, blanket weed and algae will also use nitrate for growth and one consolation when you are removing bucket-loads of the green stuff is that it represents bucket-loads of nitrate!;
Denitrification and dissimilation:
Denitrification and dissimilation are parts of another natural process that converts nitrate to atmospheric nitrogen gas. This process only occurs in the absence of oxygen. The first stage is dissimilatory nitrate reduction which reverses the nitrification process and converts nitrate (NO3–) back to nitrite (NO2–). The second stage of denitrification converts nitrite to nitric oxide, nitrous oxide and finally nitrogen gas – all of these last three products are gases that can be released into the atmosphere
NO3–
NO2–
NO
N2O
N2
It is possible to utilize this process in aquarium tanks and build denitrifying filters to remove nitrate, however, for a variety of reasons, one being the need for a constant organic carbon source, it is not practical for ponds.
The down side is that under low oxygen conditions, this process can occur naturally in a filter – especially one full of decomposing organic matter, resulting in nitrite being fed back into the system. Such a situation could and does readily happen when circumstances are right (or wrong depending which way you look at it!)
Such a scenario would be possible during very warm weather when oxygen demand was at its highest but dissolved oxygen levels at their lowest. In such circumstances during the night when oxygen levels were at their lowest because of algal respiration it would be possible for oxygen levels to fall below the critical level in a poorly maintained filter loaded with decomposing organic matter. This could occur even though the oxygen levels in the pond were acceptable. The heterotrophic bacteria in the filter, attracted by the trapped organic matter, would then switch to nitrate reduction, releasing nitrite back into the pond. The situation would be reversed at sun-up when photosynthesis restarted and oxygen was released back into the water, leaving the pond keeper blissfully unaware what had happened.
 |
To prevent nitrate reduction to toxic nitrite it is important that filters are kept clear of decomposing solids and oxygen levels are adequate, particularly during warm weather. |
So we see that nitrification is a complex, vital process and we need to be providing ideal conditions, not only for the fish, but also nitrifying bacteria.

