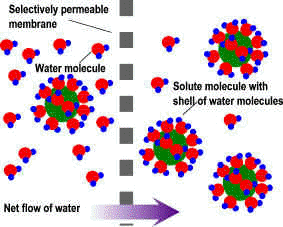
Salt, a good general disease treatment.Salt has long been the fish-keeper’s treatment standby. Salt works well against many protozoan parasites such as Costia, Trichodina and Chilodonella as well as flukes and other ectoparasites. It can also assist osmoregulation problems caused by bacterial ulcers; help clear congested gills as well as supporting fish suffering from stress. Because it works in a different way to most disease treatment, it is safer than many pond treatments and will not adversely affect biological filtration in pond filters. It is generally used at fairly high rates in short-term baths or dips, but can be used as a long-term supportive treatment in ponds. Kills parasitesIts action is based on altering the osmotic gradient between the parasite or fish and the surrounding water. Osmosis, as you will recall, is the movement of water from an area of high concentration (where the water contains less dissolved substances and therefore more free water molecules) to an area of less concentration (where the water contains more dissolved substances and fewer free water molecule). This is a difficult concept to grasp and explain, but it means that in a freshwater pond or tank there is a continuous movement of water from the pond water into the parasite’s body fluids which contain dissolved molecules such as proteins, salts, ions etc. Most aquatic organisms such as fish and parasites control this continuous influx via osmoregulation – literally regulating osmosis. (see water quality articles for more info). The details are not important as long as we understand that osmosis takes place and that in freshwater, water is being continuously drawn into fish and other organisms.
OK. That’s the difficult part – now for the clever bit. If we reverse the osmotic gradient by adding salt to the water, the osmotic flow will reverse. So now, instead of water being drawn into the parasite, it will now be drawn out, dehydrating the organism. Clever! Clearly, smaller organisms such as flukes, will be affected quicker and more severely than a large fish such as a koi. In addition to controlling parasites, short-term baths have a mild astringent effect and and help clear congested gills by lifting away cellular debris and excess mucus. Helps bacterial ulcers and stress. One of the effects of stress is to interfere with osmoregulation. A longer-term bath of a low concentration (5 grams per litre) can help alleviate osmotic stress by reducing the osmotic gradient and thus the water flow into the fish. This is particularly important in soft water because it contains less dissolved substances (and therefore has more free water molecules), and thus creates a stronger osmotic effect than hard water. Along the same lines, the same effect occurs with bacterial ulcers. Because ulcers are literally holes in the skin, water can flood into the fish’s body, again particularly in soft water. Reducing the osmotic gradient by adding a small amount of salt to the water will reduce water ingress. Treatment dosages: Prolonged immersion:
Bath treatment for freshwater ectoparasites and bacterial gill disease.
Does and don’ts
Summary: In the absence of a full examination, a salt bath is a good first choice treatment for fish that are off-colour. For a variety of reasons it is not always effective. If the fish doesn’t respond, further investigation may be required. If you don’t have quarantine facilities, salt-bathing new fish is a wise (and minimum) precaution before putting them in the tank or pond.
|