Koi are ammonia factories
Although koi are easily the most attractive and endearing of fish, when it comes to hygiene they are not quite so appealing! In fact they are incredibly mucky, often called ‘water pigs’. Their size and insatiable appetite, together with the typical stocking levels of most ponds, means that copious amounts of waste are produced every day, especially in summertime. In the huge expanse of a large lake, this isn’t a problem but the situation is markedly different in the confines of the average pond. Left alone they would quickly pollute the pond and end up poisoning themselves with their own waste! Maintaining good, clean water is the most important task for all koi-keepers, as even low levels of pollution can have serious consequences for the fish.
Nature’s help
The control of nitrogenous wastes such as ammonia and nitrite is vital, as these products can be harmful in even minute amounts. Luckily, Mother Nature, in the shape of nitrifying bacteria, provides a helping hand by breaking down these toxic wastes into less harmful products. This takes place in the nitrogen cycle, a natural phenomenon of crucial importance to koi-keeping. The nitrogen cycle is at the heart of the biological filters used by most hobbyists and it goes without saying that an understanding of the nitrogen cycle is essential if water quality problems are to be avoided. To develop a full understanding we need to appreciate the relationship between nitrogenous compounds, fish stocking levels, feeding and filtration. Then we can understand how water quality problems arise and how to rectify them before they become a danger.
Organic material usually contains proteins in variable amounts. When proteins are broken down, either by bacterial decomposition or as a waste product of protein metabolism, ammonia is formed. Bacterial action converts ammonia, which is extremely toxic, into less toxic nitrite, which in turn is converted to nitrate, a relatively harmless substance. This process of converting ammonia into nitrate is called nitrification. It all sounds great but there is potential for problems lurking in every pond if something goes wrong!
Ammonia factories
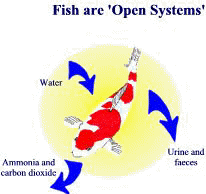
The main source of ammonia in koi ponds is fish metabolism. Metabolism is a general term covering all the various processes taking place in living organisms, such as food digestion, energy production, tissue repair and cell growth. During this process, complex food structures are broken down into simple amino acids (from proteins), carbohydrates and fats and reformed according to the fish’s needs. Any surplus is stored as body fat or excreted as waste. Excess amino acids cannot be stored and end up being converted to ammonia in a process called deamination and then excreted into the water via the gills. Any undigested food is passed out with the faeces. This process is not exclusive to fish, indeed all animals produce metabolic ammonia. However, in most animals ammonia is immediately converted into a less toxic substance, usually urea, and excreted in the urine.
In the case of fish, Mother Nature decided that the large volumes of water in oceans, lakes and rivers would adequately dilute the harmful ammonia – but obviously no one told her about koi ponds! Koi, being voracious eaters, produce large amounts of ammonia and may be considered as ammonia factories, converting expensive fish food into ammonia and other wastes. The amount of ammonia produced is directly related to the amount of food given each day, which of course depends on the stocking level and size of fish.
Further sources of ammonia are decomposing fish wastes, undigested fish food and other organic matter. When such matter decomposes, it is broken down by bacterial and fungal action into simple compounds. This process is called mineralisation. A whole host of organic products, including ammonia, are produced during decomposition, which is why rotting matter always smells so awful.
A natural difference
A good starting point in understanding the nitrogen cycle is to appreciate the difference between a natural environment, such as a lake or river, and a managed closed environment typical of the average pond. In nature, free nitrogenous compounds such as ammonia, nitrite and nitrate are extremely scarce, except where waterways are polluted by farming activities or sewage works.
The nitrate produced during nitrification is a superb plant food which plants, including algae, use to form plant proteins. Plants are then eaten by fish and other herbivores who convert the plant protein into animal protein. Predation by carnivores on the herbivores then passes nitrogen, in the form of protein, up the food-chain. When plants or aquatic animals die their protein is decomposed and released as ammonia to start the cycle over again.
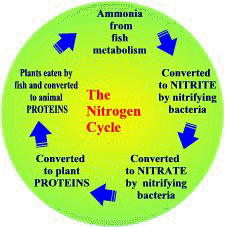 |
|
The Nitrogen Cycle |
The important point is that, in a natural system, no extra nitrogen in the form of fish food is being introduced so virtually all the available nitrogen is ‘locked away’ as plant or animal protein. Therefore high levels of ammonia, nitrite or nitrate are extremely rare.
When we consider a koi pond we see a marked difference in both stocking levels and feeding regimes, both of which combine to produce substantial amounts of ammonia. The volumes of water in a koi pond are quite minute when compared to a large lake so there is far less dilution nitrogenous compounds. The other main difference is the lack of plant life in the average koi pond. Algae, which produces ‘green water’, and blanketweed are actively discouraged by koi keepers, leading to very little nitrogen being converted into plant protein. The end result is that free nitrogenous compounds are common in koi ponds – in stark contrast to what one finds in nature.

