Water has many unusual properties that make it unlike any other liquid, some of which make it an ideal substance for aquatic habitats.
|
|
|
It takes a lot of energy to bring about a change in water temperature. On a hot day pebbles on a beach will often get hot enough to burn your feet, and it is sometimes possible to fry an egg on the bonnet of a car that has been standing in the sun! Yet the average pond will not increase in temperature by more than a few degrees, even on the hottest day.
Even on cold days, water temperature changes slowly. Without this property, water would quickly reach unacceptably high temperatures on a hot summers day and freeze solid on a cold winters day! This resistance gives aquatic environments a high degree of thermal stability, which means that fish, unlike ourselves and other warm-blooded creatures, do not have to waste valuable energy trying to maintain a constant body temperature.
Water freezes or melts at higher temperatures than its structure would suggest (certainly, when compared with a substance of similar molecular structure such as hydrogen sulphide, H2S). The atomic structure of water suggests that it should be a gas at room temperatures. Also, unlike other similar molecules, there is a large difference in temperature between the freezing and boiling points (a difference of 100oC). This makes water a secure habitat for fish because it is not constantly evaporating or freezing solid.
Ice floats but why?
Most substances get denser as they get colder. For instance, on cooling, a gas turns into a liquid and, on further cooling, the liquid becomes a solid. Thus, substances become more dense or more solid on cooling. Water is unusual in that it reaches its maximum density at about 4oC and below this temperature it becomes less dense. This has a very important consequence for outdoor fish ponds in winter. To explain this first let me remind you that things which are less dense – less solid – than water will float. (For example, a piece of wood or a duck will float but a lead weight will sink.)
Now the odd thing about water is that at temperatures below 4oC the colder water – because it is less dense – will ‘float’ to the top of the pond, leaving the denser, warmer water at the bottom. Because of this peculiarity, ice floats on water and, by forming an insulating layer on the surface, helps prevent water below from further cooling and thus freezing. (That is not to say that the water at the bottom of the pond could never freeze. For a very small, shallow pond during a hard winter it is indeed possible.) It is the warmer water at the bottom of a pond which has enabled fish and other aquatic animals to survive during severe winters. However, fish keepers should be aware of that pumps and air-stones will ‘mix’ the water and produce a near uniform temperature over the whole depth of the pond, particularly when bottom drain feeds are used. Under such conditions the bottom temperature may be just above freezing and the ‘comfort zone’ lost. During such spells water turnover should be reduced to a minimum.
Water an excellent solvent
Water can dissolve many substances, including atmospheric gases like carbon dioxide and oxygen (and fish breathe in oxygen and breathe out carbon dioxide, just as we humans do). Indeed there are few substances which do not dissolve in water to some extent. It is this property which turns water into a complex chemical cocktail and causes its composition to vary from one area of the country to another.
Why is water so unusual?
The chemical symbol for water is H20. These symbols are a shorthand which tell us that water consists of two atoms of hydrogen and one atom of oxygen. These atoms are joined together to form a molecule of water. (A molecule of water is the smallest possible bit of water, which is invisible to the naked eye, so that water as we see it consists of millions of water molecules). The water molecule is unusual in that one end of it carries a slight positive electrical charge, and the other end a negative charge, like a magnet. These charges attract water molecules to one another and causes them to stick together.
It is this “stickiness” that gives water many of its unusual properties and resistance to changes in temperature. It requires a lot of energy (heat) to break these bonds and bring about a change in temperature. In general, charged particles have only a single charge (either positive or particle having the opposite charge (opposites attract). Particles of the same charge will repel each other. But water, because it has a different charge at each end, can attract both negatively and positively charged particles. Now, for any substance to dissolve in a liquid there has to be some attraction between the substance and the liquid particles. The ability of water to attract other charged particles, regardless of their charge, gives water its superb solvent powers as well as its ability to take part in many chemical reactions.
|
|
|
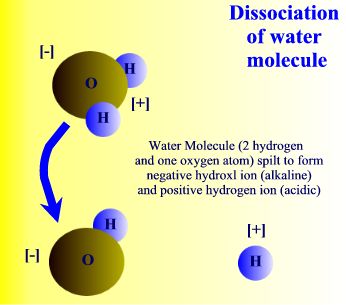
Dissociation of water molecule. |
The water molecule can also be split to form two charged particles called ions. (An ion is a general term for a particle that is either positively or negatively charged.) As explained above, water consists of hydrogen and oxygen atoms combined together, which are uncharged. But when the bond between them is broken the water molecule divides to form hydrogen ions, H+, and hydroxyl ions, OH-. These hydrogen and hydroxyl ions can take part in chemical reactions with other charged particles, thereby forming new chemicals.
So we see that water is not the inert substance that we might have imagined it to be. It has many properties which are unusual. Somewhat paradoxically, as well as providing a stable, secure environment, it is also a reactive substance and this enables it to take part in a multitude of chemical and biological reactions which play such a major part in determining the quality of the environment in a fish pond or tank.