Without osmoregulation fish will die
Osmoregulation is the control of the concentration of body fluids. If a fish is unable to regulate the effects of osmosis it will die. Clearly, osmoregulation is a vital function affecting all aspects of fish health.
Osmosis, diffusion and fish health
If we look at the structure of the gill or gut under a powerful microscope we would see the tissue that lines these structures, the epithelium, is very thin, usually only one cell thick. Such areas are rich in blood vessels with very thin walls. The combined thickness of epithelium and blood vessels is less than the size of a full stop. The structure and thinness of these areas means that the minute particles whizzing around in the water (and blood) can pass through the epithelium and blood vessels with relative ease; in fact in many cases it is as if there wasn’t any barrier at all!
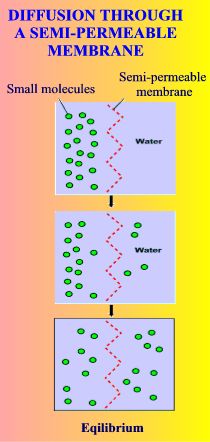 Diffusion through a semi-permeable membrane
|
A substance will diffuse from an area where it is more concentrated to an area where it is less concentrated.
Osmosis is a special case of diffusion. Water will move from an area where it is more concentrated – i.e. more pure – to an area of less concentration – i.e. containing more dissolved substances, for example more salty.
it is this difference in water ‘saltiness’ between the fish’s blood and the surrounding water that drives the constant osmosis |
If the gill and gut surfaces were unfolded and laid out flat we would have a massive area, much greater than the combined surface area of the skin and scales. At least half of the exposed surface of a fish is permeable to small particles. It is this permeability that leads us to describe a fish as an open system, constantly exchanging particles between its inside and the surrounding water. Now, perhaps, it is possible to understand why changes in the surrounding water have such an important effect on fish.
Diff and ‘Oz’
The permeability of the gills and gut and, to a lesser extent, the skin, which are all in close contact with the water, leads to a further challenge for aquatic animals. Diffusion or osmosis alone would eventually result in the fluid of a fish’s body being identical to that of the surrounding water, just as a sugar cube, left in a hot cup of tea, would eventually fully dissolve and be spread evenly throughout the tea (by diffusion). If the fluid within the fish were to be dissolved thoroughly with the water surrounding it we would, I’m afraid, have a dead fish! But the composition of the body fluids is obviously different to the pond water and has to be maintained that way if the fish is to remain alive and healthy. So how does a fish manage to do this? What is it that keeps a fish’s body fluids stable and different to the surrounding water?
Constant urination
The body fluids of a freshwater contain more dissolved salts and ions than the surrounding water. As a result of this imbalance there is a constant influx of water into its body and a loss of salts and ions from the blood outwards. A similar effect occurs if you put a dried raisin or apricot into distilled water. There is a net influx of water molecules through the skin of the dried fruit and it swells up to look like a grape again. (Try it!)
The mechanism may seem complex but essentially a fish has to rid its body of excess inflowing water by constantly excreting a weak solution of urine. Fresh-water fish can urinate approximately 30 per cent of their body mass each day. Salts are removed from the urine before it is excreted (fish are not wasters, remember) and they are also actively taken up from the water by way of the gills in order to maintain internal salt levels. This constant active absorption of salts requires energy but is essential to the fish’s survival, and anything that affects this vital function will have serious effects on the health or even survival of the fish.
The reverse situation exists in marine fish; the environment contains more dissolved salts and ions than the fish’s body so there is a net movement of water out of the fish’s body into the stronger sea-water. To replace this constant loss of water, marine fish drink sea-water and excrete the excess salts. Special cells in the gills called chloride excretory cells are involved in this process.
Clearly, any interference to the fish’s osmoregulatory systems, either fresh water or marine, would quickly prove fatal! Fresh water fish would rapidly accumulate water (a typical sign seen in dropsy), while marine fish would dehydrate. Clearly, our health considerations must take aboard the importance of osmoregulation which can be affected by instances such as water hardness, ulcer disease and stress.
To summarize, we have seen that a fish, because it lives in water, has to adapt to two major challenges, namely:
- Low levels of available oxygen,
- The need to constantly regulate and maintain the composition of its body fluids.
It is the interaction between the chemical composition of the pond water and the need to maintain constant internal conditions that results in water quality being so crucial to fish health.

