Ammonia – the silent killer
Ammonia is extremely toxic and even relatively low levels pose a threat to fish health. Ammonia is produced by fish and all other animals, including ourselves, as part of normal metabolism. Such is the toxicity, that most animals immediately convert it to a less harmful substance, usually urea, and excrete it in urine.
Fish shortcut this process and continually excrete metabolic ammonia directly into the surrounding water via special cells in the gills. In a natural environment, such as seas, lakes and rivers, it would be immediately diluted to harmless levels. However, in the confines of aquaria and ponds, levels can rapidly rise to dangerous levels unless it is constantly removed, usually by biological filtration. Additional amounts are produced from decomposing fish food, fish waste and detritus.
The effects on fish health
Raised levels affect fish health in several different ways. At low levels (<0.1 mg/litre NH3) it acts a strong irritant, especially to the gills. Prolonged exposure to sub-lethal levels can lead to skin and gill hyperplasia . Gill hyperplasia is a condition in which the secondary gill lamellae swell and thicken, restricting the water flow over the gill filaments. This can result in respiratory problems and stress and as well as creating conditions for opportunistic bacteria and parasites to proliferate. Elevated levels are a common precursor to bacterial gill disease.
Fish response to sub lethal levels are similar to those to any other form of irritation, i.e. flashing and rubbing against solid objects. Without water testing it would be very easy to wrongly conclude the fish had a parasite problem.
Gill Hyperplasia |
|
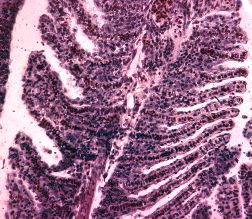 |
Fish gill with hyperplasia. The gill filaments are swollen and clumped together, reducing the fish’s ability to ‘breath’. |
|
Photo: Frank Prince-Iles |
|
At higher levels (>0.1 mg/litre NH3) even relatively short exposures can lead to skin, eye, and gills damage. Elevated levels can also lead to ammonia poisoning by suppressing normal ammonia excrement from the gills. If fish are unable to excrete this metabolic waste product there is a rise in blood-ammonia levels resulting in damage to internal organs.;
The fish response to toxic levels would be lethargy, loss of appetite, laying on the pond bottom with clamped fins, or gasping at the water surface if the gills have been affected. Because this response is similar to the response to poor water quality, parasite infestations and other diseases, it is important that a proper investigation is made to establish the real cause before administering any treatments that may exacerbate the problem.
The chemistry of ammonia
When dissolved in water, normal ammonia (NH3) reacts to form an ionised species called ammonium (NH4+)
NH3 + H2O 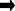 NH4+ + OH–
NH4+ + OH–
This is a shorthand way of saying that one molecule of ammonia reacts with one molecule of water to form one ammonium ion and a hydroxyl ion. From the doubled headed arrow we can tell that the reaction can go either way and hydroxyl ions and ammonium ions could combine to form ammonia and water. This is precisely what happens as the pH of water increases; that is the water becomes more alkaline. You may recall that alkalinity is caused by an increase in hydroxyl ions. An increase in hydroxyl ions (or alkalinity) pushes the equilibrium to the left and more unionised ammonia is formed.
At any given time there will be both ammonia molecules and ammonium ions present. The quantity of each species is dependant on both pH and temperature.
The toxicity of ammonia
As we have already said, ammonia (NH3) is highly toxic, whereas the ammonium ion is significantly less toxic. All test kits measure total ammonia-nitrogen (TAN), that is ammonia plus ammonium. However it is possible to determine the actual ammonia level if we know (a) TAN, (b) the water temperature and (c) the water pH.
Using this information we can then calculate the percentage of un-ionised ammonia at any given pH and temperature. Table 1 below shows how changes in pH and temperature affect the toxicity of TAN.
| Temperature oC | |||||||
| pH | 5 | 10 | 15 | 20 | 25 | 30 | |
| 6.5 | 50 | 34 | 23 | 16 | 11 | 8 | |
| 7.0 | 16 | 11 | 7 | 5 | 4 | 3 | |
| 7.5 | 5 | 3 | 2 | 2 | 1 | 1 | |
| 8.0 | 1.6 | 1.1 | 0.7 | 0.5 | 0.4 | 0.3 | |
| 8.5 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 | 0.1 | |
| 9.0 | 0.2 | 0.1 | 0.09 | 0.07 | 0.05 | 0.04 | |
|
Table 1 Showing the maximum levels of total ammonia (TAN mg/litre) for fish health |
|||||||
Table 1 shows the maximum acceptable level of TAN at a given pH and temperature. For example at pH 7.5 and a water temperature of 20oC a TAN of 2 mg / litre would be fairly safe as only 1.2% would exist as un-ionised ammonia (0.024 mg/litre). However, the danger is that should the pH or temperature rise, this ‘safe’ level would quickly become toxic!
As a rule of thumb it is best to aim for a zero level of total ammonia at all times. In normal circumstances any readings above 0.1 mg/litre TAN should be considered as unacceptable and steps taken to reduce it.
New pond or tank syndrome
Elevated levels are common when people set up new ponds or tanks. Biological nitrification is a bit of a chicken and egg situation, inasmuch that nitrifying bacteria will not multiply and grow until the ammonia or nitrite level in the water rises. Because nitrifying bacteria are slow growers it can take several weeks, even at reasonable temperatures, for the numbers to increase to a point where they can ‘process’ the ammonia through to nitrate. Until the nitrifiers are well established, ammonia, and later nitrite, levels may be unacceptable and a threat to fish health.
The first stage in nitrification is ammonia being converted to less toxic nitrite (NO2-) by Nitrosomonas sp. As the population of Nitrosomonas grows, the ammonia level starts to drop. However, this is usually followed by an increase in nitrite levels, which persists until the population of a different bacterium, Nitrobacter sp. reaches optimum levels. Nitrobacter convert nitrite to nitrate which is usually considered harmless at levels less than 50 mg/litre
Ammonia levels in a new set-up can be minimised by only introducing a few new fish at a time and allowing the nitrifiers to ‘catch up’ with the increase in ammonia before putting more fish in. There are bacterial cultures available that are supposed to speed up filter maturation, but even with these there will still be a time lag before nitrification cuts in.
How To Speed the Cycle to Almost Overnight
Problems with established set-ups.
Once a well-planted garden pond is established there are unlikely to be any ammonia problems – unless it is overstocked! However, there is always a risk of elevated levels with aquaria and koi ponds that are heavily dependant on biological filtration to maintain water quality. This can happen if;
- The filtration is inadequate for the stocking levels
- If the system is poorly maintained with large amounts of decomposing matter producing additional ammonia or inhibiting the nitrifying bacteria
- If the nitrifying bacteria have been affected by any chemical treatments or pollutants
Depending on the cause, it is vital to rectify the underlying problem by either improving the filtration or regular maintenance. In the meanwhile until zero-ammonia conditions are restored the water needs to be managed as suggested below
Reducing ammonia levels
If levels do start to increase they can be reduced by;
| Partial water changes on a daily basis until an acceptable level is obtained (How to replace water painlessly) | |
| Reduce or stop feeding | |
| Using zeolite or some other form of ion exchanging material. These act as a magnet and swap ammonium molecules in the water for another ion, usually sodium. They need to be re-charged, usually by overnight immersion in a strong salt solution. Zeolites cannot be used with salted water. | |
| It should be remembered that the aim of this management is to reduce the ammonia to an acceptable level – not zero levels, as a continuous supply of ammonia is needed to encourage the growth of nitrifying bacteria! |
Water testing
Where there is an existing problem and especially with new set-ups it is vital to test for ammonia on a frequent basis. I would suggest daily until things started to stabilise. In an established system weekly testing should be the norm. At the same time as testing for ammonia, it is important to start testing for nitrite (NO2–) as this is the next stage of nitrification, with ammonia being converted to nitrite. As the ammonia levels start to stabilise we would expect to see an increase in nitrite levels – which in turn has to be managed until the Nitrobacter bacteria that process nitrite become fully established.
