Water quality, pH and fish health.
What is pH and why is it so important to fish health and water quality? The simplest explanation is that it is a measurement of the acidity or alkalinity of a substance – in this case pond water. Changes in either can exert a powerful influence over both water quality and water chemistry, and will have a marked effect on the fish health and filter activity.
Although it determines the acidity or alkalinity of water, strictly speaking we are actually measuring the quantity or ratio of two important molecules;
- The hydrogen ion (H+), is responsible for acidity and
- The hydroxyl ion (OH–), which is responsible for alkalinity.
Some substances release hydrogen ions, or cause hydrogen ions to be formed when they dissolve in water, while others release or create hydroxyl ions.
The process of molecules splitting apart to form ions is called ionisation. Fish keepers do not need to understand the mechanics of ionisation, only to appreciate that it does happen – but if you have any queries let me know. At any time water will contain both species of ions with pH being a sort of balance sheet showing which is the predominate ion.
The pH scale
As we have already said, pH is determined by the relative quantities of the hydroxyl and hydrogen ions. The pH scale measures these ratios on a scale of 0 to 14.Very acidic solutions where the hydrogen ion predominates are measured as 0 on the scale. Very alkaline solutions in which hydroxyl ions predominate are 14 on the scale. At around pH 7, depending on temperature and salinity, the numbers of both species present are equal and therefore the water is neither acid or alkaline – it is said to be neutral.
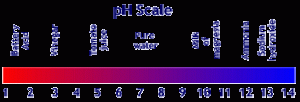 |
|
The pH of many common substances |
The simplicity of the scale can be misleading and we should be aware that the pH scale is a logarithmic measurement of the concentration of hydrogen ions, which means that each one unit change in the scale equals a ten-fold increase or decrease. Therefore 8 is 10-times as alkaline as 7, while 9 is 100-times as alkaline. So, these seemingly small changes actually represent major changes in acidity or alkalinity.
 |
Each one unit increase in pH value represents a ten-fold increase in alkalinity |
Water hardness and pH
In pure water – that is water that contains very little in the way of dissolved substances – the addition of very small amounts of acid or alkaline substances can cause quite dramatic shifts in pH. If such wide swings were to occur in ponds, lakes and the seas, for various biochemical reasons, fish and other organisms would not survive.
Luckily such fluctuations are stabilized by the presence of water-hardness causing substances. These molecules and ions act as a ‘buffers’ and ‘mop up’ any suddenly changes in the hydrogen/hydroxyl ratio. The chemistry of this, for those that are interested, is explained more fully on the water hardness page. However, as far as pH is concerned it is important that it is considered in context with water hardness as the two are closely related.
Water that is poorly buffered will be subject to more pH fluctuations than well-buffered water. As a general rule, hard water is usually alkaline and well buffered, whereas soft water is usually slightly acidic and poorly buffered.
 |
Water hardness and pH are closely connected, with pH stability dependant on on the buffering capacity of the water |
What affects pH?
Many compounds added or dissolved in water will affect the pH by adding or creating additional hydrogen or hydroxyl ions. Typically cement or concrete will make water more alkaline. By far the biggest influences are plant and animal respiration and plant photosynthesis.
All submerged plants and animals, including algae, are constantly removing dissolved oxygen from the water and excreting carbon dioxide during normal respiration. The release of carbon dioxide has an acidifying effect. In addition to respiration, during daylight hours all plants, which include all algal forms, actively photosynthesis. They absorb carbon dioxide from the water and use the sun’s energy to convert it to simple organic carbon compounds – clever!
As we have already said, carbon dioxide in solution is slightly acidic, so as the plants remove it, the water becomes more alkaline. The more sunshine and algae – the more alkaline the water will become.
These two processes, respiration and photosynthesis, carry on alongside each other, with photosynthesis being the dominant during the day. Thus during daylight hours plants have a net alkalising affect. However, during the night plants stop photosynthesis but normal respiration continues, so now they only remove oxygen from the water and excrete carbon dioxide as part of normal respiration (so much for ‘oxygenating plants’), with a net acidifying affect. In poorly buffered water this can cause significant diurnal swings (over a period of 24-hours) in pH.
Even reasonably well buffered water may have a moderate variation in pH during the day – being more alkaline in the evening. Therefore the time of day that you take you reading is important. It is also advisable to check the degree of fluctuation on a typical hot, sunny day.
The other significant factor affecting pH is nitrification, which tends to have a slight tendency to acidify water as well as removing the ‘buffering’ capacity or hardness of water.
 |
Plant photosynthesis is the main cause of high pH and diurnal pH fluctuations |
Fish health and pH
| Each species of fish has its own very narrow range of pH preference and levels outside of this range will cause health problems. For example, koi prefer a range between 7 and 8.5, while some tropical fish prefer water that is slightly acidic. There are several ways that pH can affect fish health | |
| High acidity or alkalinity can cause direct physical damage to skin, gills and eyes. Prolonged exposure to sub-lethal pH levels can cause stress, increase mucus production and encourage epithelial hyperplasia (thickening of the skin or gill epithelia) with sometimes-fatal consequences. | |
| Fish also have to maintain their own constant internal pH. Even small fluctuations of blood pH can prove fatal. Extreme external or water pH can influence and affect blood pH, resulting in either acidosis or alkalosis of the blood. | |
| The other consideration is diurnal shifts in pH, mainly as a consequence of photosynthesis as explained above. Large, fluctuations – even though they may still be within the preferred range – are likely to be stressful and damaging to health. | |
| As well as fish we should bear in mind that nitrifying bacteria in the filter also have a narrow pH range preference between 7.5 and 8.6. | |
| Changes in pH will affect the toxicity of many dissolved compounds. For example, ammonia becomes more toxic as pH increases. | |
| Variances in pH will also exert an effect on some common disease treatments, so it is important to take account of pH (and usually water hardness) when using treatments. For example, chloramine-T is more toxic in at low pH, while potassium permanganate is more dangerous at high pH. (See the treatment pages for details.) |
Changing pH
| If you have large pH fluctuations, or levels are outside the preferred range check the pH and hardness of you local water supply. If this is within the preferred range, then partial water changes over a period of time should redress the problem. | |
| If you need to physically alter the pH, use a proprietary buffer for aquaria. For ponds with a low pH – that is too acidic- use agricultural lime (calcite CaCO3 or dolomite CaMg[CO3]2) to buffer the water and increase pH. Do not use slaked lime or unslaked lime. It needs to be added over a period of a few days until the required level is reached. I would suggest some experiments in 10 litres of water to establish the amount needed for the whole pond (we are talking about several kilos normally). | |
| For ponds that have a high pH – that is the water is too alkaline, first check whether the temporary water hardness is OK. This can be measured using a simple test kit for either kH, carbonated hardness or alkalinity, which are all the same. If the water is too soft use agricultural lime as detailed above. If on the other hand the hardness is fine use either gypsum (plaster of Paris) or calcium chloride. This will improve calcium, or general hardness and stabilize pH. Substantial amounts may be needed. Some experiments in 10 litres of water will give some indication, and agricultural gypsum is available if you need large quantities. Make the adjustments over a period of several days. |
