 It is largely accepted in the hobby of koi and other types of fish-keeping that the majority of health problems relate, directly or indirectly, to water quality but it is not always understood why aquatic animals are so dependent on their environment and how changing aquatic conditions can affect their health.
It is largely accepted in the hobby of koi and other types of fish-keeping that the majority of health problems relate, directly or indirectly, to water quality but it is not always understood why aquatic animals are so dependent on their environment and how changing aquatic conditions can affect their health.
To appreciate the complexities of life in water we must first try to understand the special problems and challenges that an aquatic environment presents. There are, as we shall see, many fundamental differences between life on land and life in water, differences that can lead to major misunderstandings about fish and their needs.
It is too easy to accept the popular view that fish are somehow inferior to other animals and to see them as primitive, poorly evolved creatures. The fact that they are regarded, inaccurately, as being ‘cold-blooded’ seems to support this view and the idea that they were left behind by more highly evolved animals such as ourselves. Regrettably, the common view of them being lesser animals often leads to them being treated differently from other pet animals. Yet when we look closely we see that the similarities between fish and other animals are greater than the differences. The differences that do exist are mainly as a result of adapting to life in water.
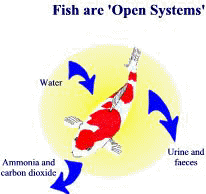
An understanding of aquatic health has to start with the relationship between fish and their environment, and a basic understanding of how they have adapted to the problems posed by life in water. Other pages will explain that water, rather than being an innate substance, is actually a complex chemical cocktail with some very strange properties. It is important to understand that even though they are solid and covered with skin and scales they are not isolated from the water they live in. Because they are dependant on their immediate environment for many vital functions they are ‘“open systems’ and as such affected by nearly every change in the surrounding water. To complete the picture of the difficulties of an aquatic existence we need to know a couple more facts about water.
A ready supply of oxygen is important to almost all animals, including fish. Air contains about 21 per cent oxygen, while water can contain only around 8 to 9 per cent at average summer temperatures. Oxygen dissolves into water slowly, and dangerously low dissolved oxygen levels can occur under certain circumstances. Also, water is 800 times denser (or heavier) than air, so we can see that living in water presents a considerable challenge for oxygen-breathing aquatic animals.
Comparing fish with higher animals, most body functions are similar and, with one important exception, the differences are minor. In common with other animals, fish use oxygen for metabolism, producing carbon dioxide as a waste product. Nutritionally, their food needs are similar to most other animals. They need protein, fats, carbohydrates, vitamins and minerals, just as we do. They have the same major organs – heart, liver, brain and kidneys etc. – and highly developed sensory organs for smell and sight, and a complex central nervous system.
So we see that fish, apart from being ectothermic and living in water, are essentially the same as most other animals. If we accept this then we can begin to appreciate the problems faced by living in water. Firstly, fish live in an environment where oxygen is only sparingly available so they must use it frugally and efficiently. Secondly, they have to cope with the effects of osmosis and diffusion and to do this requires expenditure of valuable energy.
A breath of fresh air
Animals need a constant supply of oxygen which they use in combination with food to provide energy. The availability of food and oxygen will determine the amount of energy any animal can use.
Whether an animal is warm-blooded or cold-blooded generally depends on the availability of energy sources, that is, food and oxygen. All animals have to maintain a fairly constant body temperature or their body functions can be seriously disturbed. For terrestrial animals the ability to maintain a constant internal body temperature is important because of the large fluctuations in ambient temperature that can occur. Just imagine what it would be like if your body functions came to a standstill every time the weather turned cold, you could go to the shops and not return for weeks! Despite constant changes in environmental temperature, most animals maintain a constant body temperature by consuming relatively large amounts of food and oxygen. Digestion and respiration produce energy and large amounts of heat to maintain a constant body temperature. In fact, most of the food that we eat is used just to keep our body temperature steady to within about 1oC.
The situation is different in an aquatic environment. Water has a high resistance to temperature changes so they occur slowly. So, fish are able to maintain a fairly constant body temperature without expending a lot of energy – which is just as well because oxygen and food are not so readily available.
Hopefully, we can now see that being ectothermic is not due to a lack of evolutionary development but is a necessary adaptation to a specific environment, in this case water. This adaptation means that the energy and oxygen requirements of fish are substantially less than for a warm-blooded (or endothermic) animal, such as ourselves. Fish are energy conservationists – we are comparative wasters!
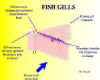
Fish make efficient use of meagre levels of available oxygen and thereby obtain sufficient oxygen to lead active lives. As we all know, fish breathe through their gills. Oxygen is removed from the water by diffusion; however, uptake is significantly enhanced by the very efficient structure and mechanism of the gills. Their large surface area and counter-current blood flow enables gills to extract up to 80 per cent of the available oxygen. Perhaps it is now obvious that anything which even slightly adversely affects the gills will seriously affect a fish’s ability to survive.
When we look at a pond or tank full of fish it is easy to think they are isolated from the water; after all, they have a skin, scales and mucus, so at least appear to be waterproof. At a microscopic level we find the opposite is true. As already mentioned, water is actually a chemical cocktail [see page “The Final Cocktail] of many dissolved substances with many added during its life in the fish or koi pond. In water, many of these substances, solids, liquids and gases, sub-divide into small particles; either molecules or ions.
These particles are very small: if we take a molecule of water for example, 2-3 million of them would fit across a full-stop on this page! In liquids and gases the particles move around at speed (faster in gases than liquids), which means that our image of pond water has to be further refined to be that of a chemical cocktail in which the minute particles of which it is composed are in constant motion.
Page One of Two > NEXT