Water hardness is, from a fish’s viewpoint, one of the most important aspects of water quality as it affects so many areas of fish health. Despite this importance to both fish health and water quality, it is often a poorly understood subject. Indeed, many fish keepers never check water hardness. This seems particularly true of koi keepers to whom it is especially relevant because of the high levels of nitrification taking place in a well-stocked koi pond.
Water hardness has a major effect on pH and pH stability. It will affect the toxicity of many common substances, including some fish disease treatments. It also has a major effect on fish osmoregulation, a process you will recall that is vital to fish health.
So what is water hardness?
As explained in “The Birth of Water”, water accumulates many dissolved substances before it reaches our taps. Hardness is a measurement of the concentration of divalent metal ions such as calcium, magnesium, iron, zinc etc, usually acquired as rainwater percolates through rock. In most water it consist mainly of calcium and magnesium salts, with trace amounts of other metals.
Two types of hardness:
The subject gets a little confusing because there are two types of hardness that we need to consider. The two types are permanent hardness and alkalinity (often referred to as carbonate or temporary hardness). The sum of both types of hardness is called the general or total hardness.
Alkalinity refers to the hardness derived mainly from carbonate and bicarbonate ions and directly reflects the buffering capacity of the water. This form of hardness is also called carbonate hardness or temporary hardness because it can be precipitated and removed by boiling the water. Which is why lime-scale forms in kettles and showerheads!
Permanent hardness measures the ions such as nitrates, sulphates, and chlorides etc, that are not removed by boiling. Most of these are not involved with buffering but can affect pH values.
In most water supplies general hardness and alkalinity measurements (as mg/litre CaCO3) are likely to be very similar because carbonates usually predominate and the amount of permanent hardness is usually fairly small.
While there is a very close connection between water hardness and buffering it should be made clear that hardness is a product of mainly calcium and magnesium ions, while buffering is produced by bicarbonate and carbonate ions. The fact that the two are so closely related is due to the fact that most hardness is formed from calcium and magnesium carbonates.
So, as a rule of thumb, hard water is usually well buffered while soft water is usually less well buffered. However, we should be aware that it is possible, because of different water composition, to have hard water that is poorly buffered, i.e water where permanent hardness predominates, or soft water that is well buffered, i.e. water that has high levels of sodium or potassium carbonate, rather than calcium or magnesium. Obviously the simple way to establish the makeup of your local water and pond water (they may not be the same) is to test for both types of hardness. Test kits are readily available for measuring both types of hardness.
The carbon dioxide/ bicarbonate/ carbonate buffering system
The initial pH of water is determined by the type of dissolved compounds that it accumulates, although it may well be chemically altered by the water company before it reaches your tap. However, once it is in the pond or aquaria, water pH is also influenced by other factors such as plant and animal respiration and plant photosynthesis. Without some form of buffering these natural activities would cause huge diurnal swings in pH.
The most common buffering system is the the carbon dioxide/ bicarbonate/ carbonate buffering system. Essentially it stabilises pH by mopping up excess hydrogen ions and then releases them again as levels drop, so that the hydrogen concentration, and therefore the pH, stays fairly constant.
CO2 + H 2O 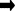 H2CO3
H2CO3 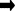 HCO – + H + CO32- (solid) + 2H +
HCO – + H + CO32- (solid) + 2H +
What this equation tells us (from left to right) is that carbon dioxide, excreted by fish and plants, dissolves in water to form carbonic acid (H2CO). If pH levels increase, that is the water becomes more alkaline (say from plant photosynthesis), then the carbonic acid dissociates to form bicarbonate and hydrogen ions (HCO3– + H+). Hydrogen ions are acidic-forming ions and will therefore counteract the alkalinity increase. If the pH continues to increase, the bicarbonate will dissociate to form solid carbonate and release yet more hydrogen ions (CO32- (solid) + 2H+), to counteract the increased alkalinity. The solid carbonate is the chalk layer covering the pond bottom and walls (or the kitchen kettle). If pH levels start to fall the process is reversed. At a normal pond pH of 7-8 some of all of the above species will be present, with bicarbonate dominating. Carbonate will predominate above pH 9.
The buffering capacity of water depends on the total amount of bicarbonate and carbonate present. Water that has low levels of these ions will quickly exhaust its ability to counteract pH fluctuations.
How much hardness?
Each fish species has its preferred range of water hardness, however it can become confusing as there are several units of measurement currently used to determine water hardness. The most commonly used method measures both alkalinity and general hardness as mg /litre of calcium carbonate (CaCO3). Another common measurement, used by Tetra, is German hardness measured as odH. These compare as seen in table 1.
|
Water |
Calcium carbonate mg / litre |
odH |
| Soft | 0-75 | 0o – 4o |
| Moderately hard | 75 – 150 | 4o – 8o |
| Hard | 150 – 300 | 8o – 16o |
| Very hard | >300 | > 16o |
| to convert odH to CacO3 multiply by 17.9 | ||
|
Table 1: Typical water hardness ranges |
||
Fish health and water hardness:
Different species of fish have varied water hardness requirements, so it is important to find out what hardness is best for your fish, For most pond fish, i.e. koi and goldfish, moderate to hard water is best. From table 1 above we can see that the optimum hardness range for most pond fish would be between 100 – 300 mg/litre CaCO3
Water hardness affects fish health because it influences osmoregulation. Being open systems, fish are affected by the makeup of the surrounding water. As a consequence of osmosis, freshwater fish are subject to a continuous influx of water, while marine fish have to live with a continuous outflow of water.
Against this continuous movement of water into or out of the body, fish have to maintain a constant internal body fluid concentration – a process called osmoregulation. The greater the difference in concentration between the fish’s body fluids and the surrounding water – the greater the osmotic effect. As hard water is more concentrated than soft, there will be less difference and therefore less water influx and consequently the fish will not have to work so hard at osmoregulation. This is particularly important in cases of bacterial ulceration where water can flood into open tissues.
Water hardness and disease treatments:
Some common fish disease treatments are affected by water hardness, and therefore needs to be considered when calculating dosages. Probably the most sensitive is chloramine-T, which is quite toxic in soft, acidic water. Copper treatments may also be hazardous in soft, acid water.
How do I change water hardness?
First because nitrification is continually removing alkalinity it is important that hardness is monitored on a regular basis – say about once a month. If either alkalinity or general hardness falls below the optimum level it can be reversed by either adding a calciferous source such as crushed oyster shell to the filter or adding more buffer to the water. If alkalinity is too low then add a carbonate buffer. If general hardness is too low then add a calcium or magnesium buffer.
